Scientific Method
- Observation- what I see, taste, touch, smell, hear
- Inference- what I guess, assume
- Prediction- a future statement with words like, “will,” “might,” “could” etc
- Hypothesis Statement- If (inference), then (prediction)
- Ex: If the grass needs light to grow, then grass in the shade will not grow well.
- Independent Variable- what I choose/change in my experiment
- Ex: The amount of light I expose the grass to
- Dependent Variable- what I measure at the end of my experiment
- Ex: The growth of the grass
- Controlled Variable- what I keep consistent in my experiment
- Ex: The type of grass, the amount of time since it was planted, the watering schedule
- Bias- my preferences that cause unfair/inaccurate results
Chemistry
- States of Matter- Solid (moves little, dense), Liquid (moves more, less dense), Gas (moves fast, low density)
- Thermal Energy- How fast or slow particles move (high T.E. = hotter, low T.E. = colder)
- Physical Property- A description of a substance that can be used to identify it (color, boiling point, solubility, etc)
- Chemical Property- A description of how a substance reacts with other chemicals or added energy (flammability, explosiveness, etc)
- Dissolve- a substance breaks into tiny pieces
- Flammability- How likely a substance is to catch fire (chemical property)
- Density- How close together particles are
- Solubility- How well something dissolves
- Exothermic Reaction- heat produced
- Endothermic Reaction- heat consumed (feels cold to the touch)
- Synthesis Reaction- Atoms form new bonds
- Decomposition Reaction- Old chemical bonds break between atoms


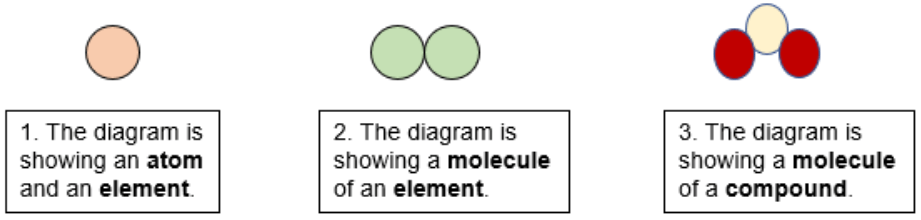
- Atom- Smallest particle of matter
- Element- Collection of purely one type of atom (pure substance)
- Molecule- More than one atom chemically bonded together
- Compound- More than one TYPE of atom chemically bonded together
- Proton- Positive subatomic particle, found in the nucleus of an atom, weighs 1 AU
- Neutron- Neutral subatomic particle, found in the nucleus of an atom, weighs 1 AU
- Electron- Negative subatomic particle, found around the outside of an atom, no AUs
- Atomic Mass- Protons + Neutrons
- Atomic Number- Number of protons (tells you which element you have, use periodic table)
- Atomic Charge- Protons – Electrons

- Balancing Ionic Equations

Metabolic Reactions
- Molecule: More than one atom bonded together
- Macromolecule: A very large molecule (our bodies cannot absorb these directly, they need to be broken up and digested)
- Macromolecules in food: Protein, Fat, Fiber, Complex Carbohydrates
- (micro) Molecules in food: Amino Acids, Fatty Acids, Glucose (and other sugars),
- Water
- Digest: Chemical bonds are broken, making molecules smaller
- Absorb: Small molecules are taken from the digestive system into the rest of the body (like a sponge absorbing water, our bodies absorb food molecules)
- Enzyme: A special type of protein that our bodies make, some enzymes digest food molecules (Amylase digests Carbohydrates into Sugar, Protease digests Protein into Amino Acids, Lipase digests Fat into Fatty Acids, humans cannot digest Fiber because our bodies cannot make the right enzyme for it)


Matter Cycling and Photosynthesis
We all depend on plants! (And plants depend on the energy from the Sun)

- Most of our food is either plant or animal. Even the animals we eat depend on plants, though. Therefore, plants are essential for all the food we eat, even if we don’t like all the vegetables we’ve tried.
- Plants have a high level of carbohydrates and sugars like glucose. Carbohydrates and glucose are molecules made of Carbon, Hydrogen and Oxygen atoms.
- Plants get Hydrogen and Oxygen atoms from the water (H2O) they absorb through roots
- Plants get Carbon and Oxygen atoms from the carbon dioxide (CO2) in the air they absorb through leaves
- Plants make their own carbohydrates and sugars using the carbon, hydrogen and oxygen they absorb AND the energy from sunlight to build new chemical bonds
Plant Anatomy



- The flower is a reproductive organ with both female and male parts.
- The pistil is the female part, with a stigma at the top and an ovary at its base.
- The stamen is the male part, where pollen is produced on the anthers. Pollen, like sperm, travels through the pistil to the ovary to create a fertilized seed.
- When the seed is fertilized, the ovary grows to become the fruit we eat (with seeds inside)


How do plants get the energy to make fruit and grow?

Photosynthesis and cellular respiration/the metabolic reaction have opposite goals. When a plant does photosynthesis, it is creating sugar to store energy from the sun in a chemical form. When a plant needs to use energy to grow, it does the cellular respiration reaction to digest sugar and release energy.
Photosynthesis starts with Carbon dioxide (CO2) breathed in from the air through stomata on the leaves. Water is sucked up from the roots into the chloroplasts. Light is absorbed by the chloroplasts and TADA! Sugar molecules (glucose) are made. In the process, extra Oxygen and water vapor is breathed out through the stomata in the leaves.
Plants and other organisms with chloroplasts can do photosynthesis- this is the only way sugar is made! Plants, animals, fungi and many types of cells can do cellular respiration- the process of digesting sugar.
